A decrease in the elasticity of the matrix contributes to the development of a number of age-related pathologies. For example, there are violations of the walls of blood vessels, damage to the tissues of the heart, cancer and neurodegenerative diseases. At the molecular level, an increase in matrix rigidity leads to impaired communication between the matrix and cells, interferes with the functioning of the immune system, and also contributes to chronic inflammation. All of these factors contribute significantly to aging throughout the body.
Aging of the matrix is primarily associated with disturbances in the main structural proteins - collagen and elastin. With age, their number decreases, the fibers become less organized, more scattered and loose. A number of factors contribute to this process, including the deterioration of the work of cells synthesizing a new matrix (fibroblasts and osteoblasts), an increase in the work of metalloproteases—enzymes that destroy the old matrix; damage caused by environmental factors such as ultraviolet light and tobacco.
With age, mechanical wear of the fibers also occurs, calcium accumulates in them, oxidation by reactive oxygen species occurs. However, according to recent studies, structural changes in collagen and elastin fibers, associated with the formation of crosslinks - chemical bonds that connect different fibers together, make the greatest contribution to matrix aging. Such crosslinks can act as molecular “handcuffs” by binding adjacent proteins and preventing them from moving independently.
An excess of cross-links leads to a violation of elasticity, and also complicates the renewal of the matrix, since the fibers cross-linked with each other become less accessible for cells and enzymes involved in its renewal.
Crosslinking in the matrix
Collagen and elastin are long-lived proteins. The half-life of collagen (the time it takes for half of the pool of molecules to be broken down) in some tissues is more than a hundred years. And in the femoral cartilage and in the intervertebral discs it even reaches 200. This means that such proteins are especially sensitive to the accumulation of damage, since the process of their renewal occurs at a low rate and the damaged proteins remain in the body for many years.There are several mechanisms for the formation of crosslinks in matrix proteins:
- Enzymatic formation of cross-links using transglutaminase and lysyl oxidase enzymes. This process normally occurs during the maturation of the matrix fibers, since a certain amount of crosslinks is necessary to stabilize the fibers. However, with age, there is an excessive activation of these enzymes, which contributes to increased tissue stiffness.
- Non-enzymatic crosslinking does not require enzymes to work and is much more critical to the aging process. It constantly occurs throughout life and (unlike enzymatic), is chemically irreversible. Most often it occurs through glycation.
Glycation
Glycation is the attachment of a simple sugar (glucose, fructose, or derivatives) to a protein to form chemical crosslinking. This reaction occurs spontaneously and does not require enzymes to work. In simple terms, this is the reaction of "adhesion of sugars to proteins." We often see it in everyday life, when, when baking, a crust is obtained on a kebab or bread. However, without additional "frying" it also proceeds, just more slowly—this is exactly what happens in our body.The chemical name for this reaction is the Maillard reaction, and the substances on the crust of bread are called glycation end products, or AGEs. Finite in the sense that the reaction is irreversible, and the crust will never turn back into raw bread. The presence of AGE in our body is a serious problem, since proteins fused with sugars cease to perform their functions, and they can no longer return to their normal functional state. AGE is garbage that must be disposed of, as their accumulation leads to a number of pathologies, which causes an increase in the level of inflammation in the body.
Glycation can be thought of as a kind of chemical “wear” of proteins. This is a random, stochastic process that takes place in our body without any genetic program. Sugars (glucose, fructose, galactose) are the sources of energy for all cells, and their presence in the blood is necessary for the vital activity of the body. Thus, glycation can be viewed as a by-product of the presence of sugars in the blood.
With age, glycation processes lead to a variety of pathological consequences. Glycation is the cause of most tissue damage in diabetes mellitus; can lead to dysfunction of mitochondria; accelerating the process of neuronal degeneration in Parkinson's disease;
an increased content of AGE is found in the nerve cells of patients with Alzheimer's disease.
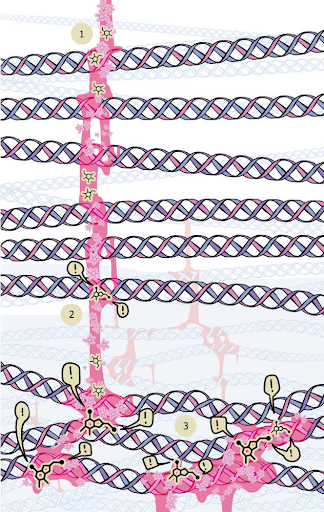
Figure 1: schematic representation of the protein glycation process: non-enzymatic attachment of monosaccharides (1) to the free amino group of the protein (2). Exclamation marks (!) denote the places where stitches were formed.
From a chemical point of view, the Maillard reaction occurs in several stages. At the first stage, sugar attacks the free amino group of lysine or arginine (which is part of the protein) with the subsequent formation of the Schiff base. Then the Amadori product is formed, which, in the course of several transformations, turns into dicarbonyls, leading to the formation of crosslinking. It is important to note that at the first stages the reaction is reversible, however, at the final stages (during the formation of AGEs with a cyclic structure), not a single enzyme of the body can break them down.
Various types of AGEs can be formed as a result of the Maillard reaction. The most common of these are collagen modifications such as glucosepane, pentosidine, carboxyethyl lysine, crosslin, vesperlysine, and MOLD. The type of AGE depends on many factors, for example, on the source and type of the carbonyl group of the sugar (which attacks the amino group of the protein in the early stages of the Maillard reaction); the type of amino acids as well as the metabolic state of the individual. For example, for the formation of glucosepane, glucose, lysine and arginine are involved in the reaction.
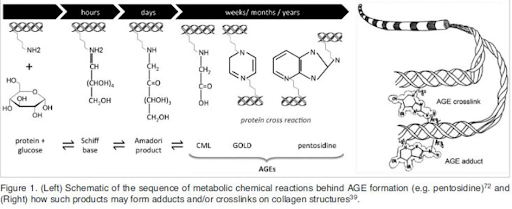
Figure 2. Left: Schematic representation of the Maillard reaction, during which the formation of AGEs (for example, pentosidine) occurs. Right: Demonstration of how AGEs can form crosslinks and/or adducts on collagen structures.
It is important to note that AGEs are already used in clinical practice as a diagnostic marker for a number of diseases. For example, glycated high and low density lipoproteins can accurately predict the development of coronary heart disease, as well as the appearance of symptoms of atherosclerosis. The content of glucosepane in the skin (one of the most common AGEs) is used to diagnosecardiovascular diseases and diseases of the nervous system.
In general, the most promising direction in the development of predictive diagnostics of protein glycation is the combination of a large number of different analyzes in combination with machine learning techniques. Already, such approaches are showing their first results in the diagnosis of autism.
Matrix crosslinks as a pathway to aging
The stochastic formation of crosslinks leads to an age-dependent accumulation of damage in matrix proteins. In the childhood period, such injuries are invisible, since they are compensated for by new actively synthesized proteins of the intercellular substance and an increase in the volume of the whole organism. However, after puberty and growth arrest, the accumulation of crosslinks begins to contribute more and more to the decline of health in the body.The nature of the formation of cross-links lies in the area of non-enzymatic combination of proteins with sugars coming from the blood. At the same time, the human body does not have enzymatic systems that allow it to disconnect the crosslinks from the proteins of the intercellular substance. The only available way is to disassemble the entire protein macromolecule by matrix collagenases or proteinases. But this is an energy-consuming, labor-consuming and slow process, which does not allow compensating for all the resulting crosslinks.
Moreover, the efficiency of collagenase degradation of uncrosslinked collagen is significantly higher than that of damaged proteins. Thus, enzymes first begin to destroy the least cross-linked collagens, and only destroy abundantly glycated ones at the end. As a result, the amount of collagen and elastic fibers in the old matrix decreases, and those that remain are highly glycated. The fragility and rigidity of the fibers of the matrix increases.
The interaction of the old matrix with the surrounding cells leads to a number of pathological processes, including a decrease in stem cell division, impaired cell migration and intercellular signaling, as well as chronic inflammation, which ultimately stimulates the emergence of a wide range of age-related pathologies and contributes to aging in general.
An important aspect of matrix aging is the slow speed and multistep process of transformation of the sugar molecule and amino acids into the final and chemically stable AGE molecule. At various stages of this process, such as the stage of formation of the product of Amadori and Schiff bases, the occurrence of future crosslinking can be prevented pharmacologically by anti-glycation agents. Their development is one of the most important tasks of modern gerontology.
